Using Brain Imaging to Unravel the Mysteries of Stuttering
By Soo-Eun Chang, Ph.D.
[1]August 23, 2011
From the Dana Foundation [2]
Editor’s note: After many decades of attributing stuttering to causes ranging from childhood trauma to overly anxious personalities, scientists have used neuroimaging techniques to uncover measurable differences in the brain activity of people who stutter versus fluent speakers. But while researchers have made great strides in understanding stuttering in adults, the neural basis of stuttering in children largely remains a mystery. We do not yet know why up to 80 percent of children who stutter recover without intervention, nor do we know how to distinguish those who will recover without intervention from those who will not. However, recent findings support the idea that early intervention can alter or normalize brain function before stuttering-induced changes become hardwired.
As many as 5 percent of children between ages two and five stutter, usually beginning around the time they start forming simple sentences. In addition to exhibiting well-known speech symptoms such as repetitions, blocks, and prolongations that primarily occur in initial sounds or syllables of words and sentences, children who stutter may also experience physical symptoms, such as eye squinting, neck and face tensing, and arm and leg movements that can be distracting to the listener. In the United States alone, an estimated 3 million people stutter.
Considering the high incidence of stuttering, we know very little about its etiology. People have attributed stuttering to numerous and various possible causes, such as childhood trauma (as is suggested in the movie The King’s Speech), hypercritical parents, or an overly anxious personality, none of which have been supported in the scientific literature.
We also do not know why many children grow out of stuttering within a few years of the onset of symptoms, while others continue to stutter for the rest of their lives. There is no objective marker that can help us discern which children will recover and which will develop chronic stuttering. Because up to 80 percent of children who stutter recover spontaneously, the usual recommendation to a concerned parent used to be to wait and see. The conundrum is that waiting could be disadvantageous to children who could benefit from early intervention. Today, most fluency specialists recommend that parents consider speech-language therapy if a child has been stuttering for more than six months, particularly if the child finds it bothersome. When making a decision regarding intervention, specialists may also consider additional factors such as age of stuttering onset, sex, family history of persistent stuttering, and phonological (speech sound) development.
In this article I review some recent advances in determining the neural bases of stuttering and discuss why early intervention may be important in the context of brain development. With the advent of neuroimaging, scientists now have the unprecedented ability to use sophisticated techniques to examine the anatomy and functions of living brains. What we now know, based on neuroimaging research, is that people who stutter and people who speak fluently exhibit clear differences in brain-activity patterns during speech production. In addition, people who stutter exhibit subtle structural deficits, primarily involving left-hemisphere brain regions that support fluent speech production. In the future, researchers might develop therapies that maximize brain plasticity conducive to producing fluent speech. I also discuss how we may find objective markers of chronic stuttering, which could lead to the development of better treatments for this complex condition.
Fluency-Inducing Conditions and the Neural Bases of Stuttering
Many people who stutter report that their stuttering goes away completely in certain situations, such as when they speak to their children or to a pet, sing, talk in chorus with others, or even adopt a novel manner of speaking (for example, speaking with an accent or speaking as an actor onstage). Speaking under delayed auditory feedback, which delivers an “echo” of one’s voice while speaking (one hears back one’s voice with a split-second delay), or frequency-altered feedback, which delivers feedback of one’s voice with a pitch shift (one hears one’s voice altered to be either higher or lower in pitch), can also induce fluency in many people who stutter. The fact that people who stutter often show a dramatic decrease in stuttering during an altered-feedback condition—one that usually disrupts speech in fluent speakers—suggests that the auditory and motor centers of the brain interact differently in this group relative to fluent speakers. In addition, many of the fluency-inducing conditions promote slowed rates of speech and provide externally delivered timing cues for speech movement. These conditions may compensate for a speech system that is less able to sequence speech movements rapidly and perhaps unable to rely on internal timing of speech movements.
Data from recent neuroimaging studies on stuttering give us insights into the possible bases of these fluency-inducing conditions in stuttering speakers. The main brain regions that work together to make fluent speech production possible include areas in the frontal cortex of the brain, which controls movement planning and execution, and auditory sensory regions located farther back, in the temporoparietal cortex. Regions deeper within the brain, including the basal ganglia, thalamus, and cerebellum, also support speech movements by providing internal timing and sequencing cues. It is in these brain regions and their connections that researchers have found brain function and anatomy differences between stuttering speakers and fluent speakers.
Evidence of Aberrant Auditory-Motor Integration
Fluid, effortless speech production is possible because of well-established connections among brain regions that support auditory processing, motor planning, and motor execution. These connections become established when a child learns to speak by matching the sounds that he has heard in a model’s, such as a mother’s, speech with sounds generated by his own speech movements. With practice, the child’s speech sounds begin to match the targeted speech sounds. According to one speech model, the auditory cortex, which houses the auditory representation of speech sounds, is connected with speech planning and execution areas.1 This connection is achieved through a dorsal stream that researchers posit to be much more highly developed in the left hemisphere. Researchers claim that the dorsal stream anatomically corresponds to the superior longitudinal fasciculus, a major white-matter pathway that connects the brain structures located in the anterior (motor) and posterior (sensory) parts of the brain.2 The white-matter tracts act like electric cables, transmitting nerve impulses from one part of the brain to another. If the integrity of these white-matter tracts is compromised, the rapid information exchange that needs to occur among the major areas that support speech may also be compromised.
Some neuroimaging data support the idea that people who stutter may have aberrant connections relative to fluent speakers, primarily in the left hemisphere that involves a major white-matter tract (figure 1). In this white-matter pathway, the superior longitudinal fasciculus connects the brain regions involved in speech planning in the inferior frontal region with the auditory regions involved in the sensory feedback of speech sounds, via the motor cortex, which is responsible for speech-motor execution (figure 2). Studies have reported subtle decreases in white-matter integrity in the left superior longitudinal fasciculus in both children and adults who stutter.3-6
--------------------------------------------------------------------------------
Figure 1
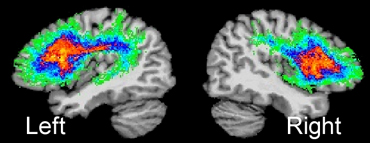 |
Figure courtesy of Soo-Eun Chang
The superior longitudinal fasciculus (SLF) in the left and right hemispheres. SLF is a major white-matter tract that interconnects several brain regions important for speech production. Here, SLF is shown for the left and right hemispheres based on 14 normally fluent individuals.46 The left SLF is greater in fiber tract density compared to the right SLF,47 which underscores its role in supporting speech and language function.
Figure 2
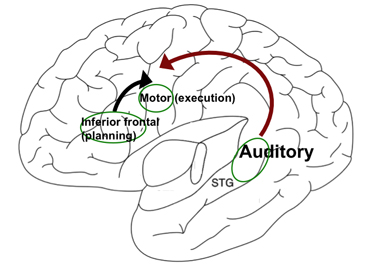 |
Figure courtesy of Soo-Eun Chang
A simplified model of the left hemisphere showing the inferior frontal region (speech planning), motor cortex (speech execution), and superior longitudinal fasciculus (auditory processing), which is interconnected via the superior longitudinal fasciculus (depicted with arrows).
--------------------------------------------------------------------------------
According to some studies, stuttering speakers have greater volume and activity in the right side of the brain compared to the left side, perhaps as a reaction to the left-sided connectivity deficits. Nonstuttering adults, in contrast, have greater left- than right-side auditory cortex volume. Moreover, stuttering adults with the greatest rightward asymmetry (right greater than left) brain volume in an auditory association region exhibited more severe stuttering and experienced the greatest benefit from delayed auditory feedback during speech production.7
Researchers who examined brain-activity patterns in adults who stutter during various speech-production tasks found underactivity in the auditory cortex and overactivity in the motor regions. Relative to the nonstuttering control group, stuttering speakers exhibited heightened activity in the right hemisphere in motor regions8-10 as well as in the cerebellum11 and lowered activity in the auditory areas. In conjunction with abnormal anatomy in these regions, and particularly in the left hemisphere, this right-sided overactivity might be explained as a compensatory reaction to the left-sided deficit in the auditory areas.
Brain Development in Children Who Stutter
Studies of children who stutter are critical. The neural correlates of stuttering are difficult to establish when examining only adults, since people who stutter for decades may develop compensatory mechanisms that have become hardwired in the brain. These compensatory effects—which are much less likely in the brains of children—may confound the core deficit associated with stuttering. As it turns out, all studies discussed above involved adult participants, mostly due to the practical challenges of conducting neuroimaging research on young children.
Researchers have conducted large-scale studies examining typical brain development in children, however. These studies show that brain structures supporting speech and language development have a protracted growth pattern relative to other areas of the brain (such as vision).12-14 Researchers found that the superior longitudinal fasciculus continues to develop even up to adolescence.15,16 Thus, during the course of speech acquisition, it is possible that structures supporting speech production may develop differently in children who stutter compared to children who speak fluently. In addition, the development of these structures and the connectivity among them may differ in children who recover from stuttering compared to those who continue to stutter into adulthood. Given that in typical brain development, these structures particularly maintain plasticity into later childhood and adolescence, the discovery of differences may also have significant implications for therapy that results in lasting recovery from stuttering.
Brain Anatomical Differences in Children Who Stutter
In the only published study to date on the neuroanatomical bases of childhood stuttering, we compared children with persistent stuttering, children who recovered naturally from stuttering, and age-matched fluent controls on several different brain structure measures. All 21 children who participated were 8- to 12-year-old, right-handed boys.3 We examined both differences in the integrity of white matter (the tracts that connect different areas in the brain) and differences in the volume of gray matter (composed of nerve-cell bodies and dendrites, where information processing takes place) among the groups.
We found evidence of decreased white-matter integrity in the superior longitudinal fasciculus underlying the sensorimotor cortex in stuttering children relative to age-matched controls. A decrease in white-matter integrity in this area may mean that signals among the movement planning, execution, and sensory brain areas may not be transmitted in a sufficiently rapid manner to allow for fluent speech production. This decrease was common for both those who were persistent stutterers and those who had recovered from stuttering. Interestingly, the recovered group showed an intermediate level of white-matter integrity, between that of the persistent stuttering and control groups. Additionally, the recovered children showed trends of increased white-matter integrity in the right-hemisphere homologue region, the equivalent region in the right hemisphere that mirrored the left hemisphere region found to have less integrity in stuttering children. These findings warrant confirmation with larger groups to determine whether brain areas showing distinct growth in recovered children (as found in this study) underlie natural recovery.
Our study replicates findings from an earlier study on stuttering adults. That study found that left-sided white-matter integrity decreases in the sensorimotor cortex region in adults who stutter compared to controls.4 The left-sided decrease in white-matter integrity found in adults—and now in children—clearly suggests that this may be one of the important structural bases for stuttering. Although the school-age children who had persistent stuttering had been stuttering since two to four years of age and were likely to have adopted some compensatory behaviors similar to those of adults, this cannot be said about the age-matched group of children who had recovered from stuttering and who had not been stuttering for at least two years prior to their study participation. The fact that both groups exhibited the same white-matter differences compared to the control children suggests that this structural difference may be associated with the risk of developing stuttering, regardless of outcome. In addition, our study reported significant differences in white-matter integrity between children with a stuttering history (both persistent and recovered) versus fluent children in an area that contains thalamocortical and corticonuclear tracts. These tracts connect cortical brain regions with subcortical areas and cranial nerves, respectively, that can directly control speech musculature. If these connections are affected, coordination of speech musculature allowing adequate timing, amplitude, and sequence manipulation that is typical of fluent speech could be affected as well.
Right-hemisphere brain volume increases previously reported in adults who stutter17,18 were not found when examining gray-matter volume in children who stutter.3 Children who stutter exhibited the typical leftward asymmetry in gray-matter volume, particularly in the posterior temporal cortices (auditory association areas). This suggests that right-hemisphere enhancement may develop with continued stuttering into adulthood. Perhaps the auditory cortex increases found in children with persistent stuttering are the result of continuing to stutter for six to nine years after onset.3
In summary, children who stutter, regardless of whether they continue to stutter or recover from stuttering, appeared to have brain connectivity differences when compared to their nonstuttering peers. The differences suggested that dynamic and timely interactions among the left motor cortical and sensory regions may be affected in children who stutter, thus resulting in nonfluent speech. All children, including both of the stuttering groups, exhibited the normal left-greater-than-right asymmetry pattern. This suggests that the increased right-sided volume found in adults who stutter could be the result of compensation for aberrant left-hemisphere connectivity. Because this study was based on relatively small numbers of children and the persistent children were more than two years past the onset of stuttering symptoms, it is important to replicate these findings in larger groups and in younger children closer to stuttering onset. In addition, this study examined only boys who stutter; considering the skewed sex ratio in stuttering (for every girl who stutters, there are five to seven boys who stutter) and the fact that most girls who stutter recover from it, it would be important to examine both gender groups in future studies.
Brain Function Differences in Children Who Stutter
The anomalous anatomical growth reported in children who stutter may impact how brain regions interact when producing speech. In turn, sustained anomalous function could lead to further structural changes in the brain. To date, researchers have conducted only a few studies examining differences in brain function in young children who stutter.
Conducting neuroimaging studies with children presents many practical challenges. Any study that uses magnetic resonance imaging (MRI) or functional magnetic resonance imaging (fMRI), for example, requires restriction of head movement; children must stay immobilized in a small space under loud noise during scanning. Other techniques, such as positron-emission tomography (PET), involve injecting radioactive substances, which are inappropriate to use in children without a clinical justification.
Perhaps reflecting these practical challenges, studies measuring brain function in stuttering children have so far been limited to using event-related potentials, or ERPs. These studies involve measuring stereotypical electrophysiological responses to a given stimulus (such as auditory presentation of a tone or a vowel) via an electroencephalogram (EEG) or magnetoencephalogram (MEG). Using electrodes or very sensitive coils along the scalp, EEG and MEG can pick up electrical and magnetic field potentials, respectively, which are associated with neural activity. Both methods can capture the brain responses of interest almost as soon as they occur. However, the spatial resolution, which relates to localizing the brain activity to a certain region of the brain, is much less reliable than other neuroimaging methods such as fMRI.
An ERP study conducted with school-age children who stutter reported that stuttering children were significantly less accurate than controls when making rhyming judgments that required phonological rehearsal. The authors noted that the brain’s evoked responses related to the cognitive processes preceding this task were altered in children who stutter, and that the responses peaked earlier in the right hemisphere than in the left, while the brain responses peaked earlier in the left than the right in the controls.19 The same research group conducted another ERP study on preschool-age children who stutter and found that children who stutter lacked a characteristic waveform that is typically elicited in normal children in response to deviant auditory stimuli. This indicated aberrant cognitive mechanisms involved in processing auditory stimuli, even in the youngest stuttering children.20
Another study examining school-age children who stutter used MEG to examine a well-known phenomenon that illustrates the interaction between speech motor and auditory areas: vocalization-induced suppression.21 The auditory cortex is normally inhibited during vocalization, unlike when we listen to a recording of the same vocalization. According to scientists, this phenomenon underscores the tight collaboration between the auditory and motor regions of the brain to enable normal speech production. The researchers measured the brain’s evoked responses to listening to a tone, listening to a vowel, and producing a vowel in school-age children who stutter. The children did not differ from age-matched controls in their evoked response to simply listening to the tone, but they did differ in their response to vowel perception and production. The amplitude of the evoked responses did not differ, but the latency of response was delayed in both hemispheres of children who stutter.
In the most recently published study, the extent of laterality (left versus right cerebral dominance) in brain function for phonological and prosodic contrast tasks was reported in adults, school-age children, and preschool-age children who stutter.22 The phonological task involved perceiving differences in distinct units of speech sounds, while prosody contrasts involved perceiving differences in intonation. The authors expected that speech sounds, compared to intonation changes, would be perceived better in the left hemisphere compared to the right, as the former involves linguistic processing, which lateralizes to the left hemisphere in the vast majority of individuals. Using near infrared spectroscopy, a method that allows noninvasive examination of brain function similar to fMRI and PET, but is less restrictive for young participants, the researchers found that age-matched nonstuttering speakers consistently exhibited greater left than right laterality of brain response when listening to auditory stimuli differing in phoneme versus prosody. In contrast, not even one subject among the stuttering group exhibited leftward laterality for the phoneme versus prosodic contrasts. This was true for all age groups, including the youngest preschool-age children. The researchers speculated that due to left-sided anatomical deficiencies, both linguistic and prosodic functions may lateralize to the right hemisphere in stuttering children, and as this pattern is maintained, children may display right-sided structural increases, as have been reported in anatomical studies of adults who stutter.7,17,23
Current data point to differences in brain function and anatomy, involving both auditory and motor areas of the brain, even in the earliest stages of stuttering. The functional brain differences in stuttering children, when sustained, could result in structural brain changes, in turn resulting in abnormal laterality of auditory-motor interaction for speech processing—which is reported in stuttering adults. Future studies that track both functional and structural brain growth as stuttering children develop are likely to give us more definitive answers on a number of still-unanswered issues, such as why some children naturally recover from stuttering and why many more girls grow out of stuttering than boys.
Implications for Treatment
Currently there is no cure that works for all people who stutter. At present, behavioral therapy by a skilled speech-language pathologist (ideally someone who specializes in fluency) is the most viable option for treating stuttering. Although much more data is needed before direct clinical applications can be made, there is support for early intervention for children who stutter. If parents are concerned about their child’s stuttering, and the child has been stuttering for more than six months, therapeutic intervention should be considered. The brain regions found to be different in stuttering children are primarily those that undergo active growth and are plastic during childhood, and are thus more likely to respond to treatment that stimulates brain development toward more normal growth patterns. It is probable that there is greater chance of lasting recovery if therapy is delivered during early childhood rather than after adolescence. In the latter case, the stuttering speaker may still benefit (as King George VI did), but he may need effortful monitoring of his speech to achieve fluency, and there is possibility of relapse.
Experienced clinicians claim that successful treatment of children often takes less time than is necessary for adults, and normal fluency is the goal for most children. For these children, recovery may occur either because they adopt a compensatory neural growth pattern that successfully makes up for the deficient brain regions, or because they are able to adopt a pattern of development that resembles normally fluent children (we do not at present have any evidence to substantiate either of these claims).
If a child continues to stutter into adolescence and beyond, the window of dynamic growth in the speech regions supporting fluent speech may close; an adult is likely to be much more resistant to change. Reflecting these ideas, the goal for most adult therapeutic interventions is not normal fluency, but rather a state in which stuttering occurs with less tension (stuttering modification) or a speech pattern that is volitional and consciously controlled due to relearning the components of fluent speech, including respiration, phonation, and articulation (fluency shaping). These modified speech patterns are different from the effortless and automatic speech production that is typical of normally fluent speakers. Treatment in adults must also address psychosocial issues, which are less commonly seen in early childhood stuttering. Adults who stutter are likely to have developed an emotional reaction to their stuttering, and many exhibit avoidance behavior associated with speech situations, which can exacerbate and perpetuate stuttering.
Several groups have studied brain changes associated with stuttering treatment (primarily fluency shaping) during adulthood.11,24-26 Some of the major findings indicate that therapy leads to attenuation of right-hemisphere overactivity seen before therapy, as well as a shift toward more activity in the left hemisphere regions supporting planning, execution, and auditory feedback of speech. Abnormal basal ganglia activity also decreased following therapy.27 These brain changes are still very different from brain activity we see during speech of fluent individuals, however—an indication that at least at the neural level, there are limits to what adults who stutter can achieve through therapy. These findings all the more support the idea that early intervention may be important, as therapy during early childhood provides an opportunity to alter or normalize brain function before stuttering-induced changes become hardwired and perhaps less responsive to therapy.
In the future, researchers should examine therapy’s effects on children and determine whether therapy-induced recovery during early childhood leads to similar brain function and structure as found in children who have recovered or children who have never stuttered. If the therapy-induced brain changes do not lead to brain structure and function that resemble normal brain growth in children who never stuttered, yet the children who once stuttered achieve full recovery without relapse, this may indicate a successful compensatory growth that may be a goal of future behavioral treatment for both children and adults.
Advances in genetics research may lead to better understanding of the molecular basis and biological pathways associated with stuttering28-30 and, eventually, to better diagnostic and treatment approaches including pharmacological treatment* and gene therapy. There is substantial evidence that genetic factors contribute to stuttering. Stuttering shows a strong familial aggregation,33-35 and twin studies have shown that there is greater concordance for identical twins than for fraternal twin pairs.36-38 While it is certain that there is a strong genetic contribution to stuttering, the mode of inheritance is still unclear. Several genome-wide linkage studies39-43 have indicated only moderate evidence of linkage to any one region, and replication of results across the different labs has been sparse. The recent discovery of mutations of specific genes associated with lysosomal dysfunction (dysfunction of the cell organelles that break down cellular waste matter and debris) in stuttering families44 has been suggested as a possible neurochemical basis for the white-matter deficits,45 but the results await replication by independent groups. More research must confirm the relationship between the genetic mutations and brain development patterns relevant to stuttering.
We are still at an early stage of understanding the basis for this enigmatic speech condition. With more advances in the study of the neural bases and genetics of stuttering, scientists may find an objective biological marker for persistent stuttering, as well as brain changes that lead to successful recovery from stuttering. These future developments will lead to better clinical assessment and bring us closer to finding treatment targets. As we near the discovery of the etiology of stuttering, we will be closer to finding a long-term cure.
--------------------------------------------------------------------------------
*Recently a large-scale clinical trial was conducted on a drug called pagoclone to treat stuttering in adults;31 more studies are needed to establish the reliability of the results.32
References
1. Hickok, G., & Poeppel, D. (2007). The cortical organization of speech processing. Nature Reviews Neuroscience, 8(5), 393-402.
2. Saur, D., Kreher, B. W., Schnell, S., Kummerer, D., Kellmeyer, P., Vry, M. S., . . . Weiller, C. (2008). Ventral and dorsal pathways for language. Proceedings of the National Academy of Sciences, USA, 105(46), 18035-18040.
3. Chang, S. E., Erickson, K. I., Ambrose, N. G., Hasegawa-Johnson, M. A., & Ludlow, C. L. (2008). Brain anatomy differences in childhood stuttering. Neuroimage, 39(3), 1333-1344.
4. Sommer, M., Koch, M. A., Paulus, W., Weiller, C., & Buchel, C. (2002). Disconnection of speech-relevant brain areas in persistent developmental stuttering. Lancet, 360(9330), 380-383.
5. Watkins, K. E., Smith, S. M., Davis, S., & Howell, P. (2008). Structural and functional abnormalities of the motor system in developmental stuttering. Brain, 131(Pt 1), 50-59.
6. Cykowski, M. D., Fox, P. T., Ingham, R. J., Ingham, J. C., & Robin, D. A. (2010). A study of the reproducibility and etiology of diffusion anisotropy differences in developmental stuttering: a potential role for impaired myelination. Neuroimage, 52(4), 1495-1504.
7. Foundas, A. L., Bollich, A. M., Feldman, J., Corey, D. M., Hurley, M., Lemen, L. C., & Heilman, K. M. (2004). Aberrant auditory processing and atypical planum temporale in developmental stuttering. Neurology, 63(9), 1640-1646.
8. Braun, A. R., Varga, M., Stager, S., Schulz, G., Selbie, S., Maisog, J. M., . . . Ludlow, C. L. (1997). Altered patterns of cerebral activity during speech and language production in developmental stuttering. An H2(15)O positron emission tomography study. Brain, 120 ( Pt 5), 761-784.
9. Fox, P. T., Ingham, R. J., Ingham, J. C., Hirsch, T. B., Downs, J. H., Martin, C., . . . Lancaster, J. L. (1996). A PET study of the neural systems of stuttering. Nature, 382(6587), 158-161.
10. Chang, S. E., Kenney, M. K., Loucks, T. M., & Ludlow, C. L. (2009). Brain activation abnormalities during speech and non-speech in stuttering speakers. Neuroimage, 46(1), 201-212.
11. De Nil, L. F., Kroll, R. M., & Houle, S. (2001). Functional neuroimaging of cerebellar activation during single word reading and verb generation in stuttering and nonstuttering adults. Neuroscience Letters, 302(2-3), 77-80.
12. Sowell, E. R., Peterson, B. S., Thompson, P. M., Welcome, S. E., Henkenius, A. L., & Toga, A. W. (2003). Mapping cortical change across the human life span. Nature Neuroscience, 6(3), 309-315.
13. Sowell, E. R., Thompson, P. M., Leonard, C. M., Welcome, S. E., Kan, E., & Toga, A. W. (2004). Longitudinal mapping of cortical thickness and brain growth in normal children. Journal of Neuroscience, 24(38), 8223-8231.
14. Lu, L. H., Leonard, C. M., Thompson, P. M., Kan, E., Jolley, J., Welcome, S. E., . . . Sowell, E. R. (2007). Normal developmental changes in inferior frontal gray matter are associated with improvement in phonological processing: A longitudinal MRI analysis. Cerebral Cortex, 17(5), 1092-1099.
15. Paus, T. (1999). Structural maturation of neural pathways in children and adolescents: In vivo study. Science, 283(5409), 1908-1911.
16. Giorgio, A., Watkins, K. E., Douaud, G., James, A. C., James, S., De Stefano, N., . . . Johansen-Berg, H. (2008). Changes in white matter microstructure during adolescence. Neuroimage, 39(1), 52-61.
17. Foundas, A. L., Bollich, A. M., Corey, D. M., Hurley, M., & Heilman, K. M. (2001). Anomalous anatomy of speech-language areas in adults with persistent developmental stuttering. Neurology, 57(2), 207-215.
18. Jancke, L., Hanggi, J., & Steinmetz, H. (2004). Morphological brain differences between adult stutterers and non-stutterers. BioMedCentral Neurology, 4(1), 23.
19. Weber-Fox, C., Spruill, J. E. III, Spencer, R., & Smith, A. (2008). Atypical neural functions underlying phonological processing and silent rehearsal in children who stutter. Developmental Science, 11(2), 321-337.
20. Kaganovich, N., Wray, A. H., & Weber-Fox, C. (2010). Non-linguistic auditory processing and working memory update in pre-school children who stutter: an electrophysiological study. Developmental Neuropsychology, 35(6), 712-736.
21. Beal, D. S., Quraan, M. A., Cheyne, D. O., Taylor, M. J., Gracco, V. L., & De Nil, L. F. (2011). Speech-induced suppression of evoked auditory fields in children who stutter. Neuroimage, 54(4), 2994-3003.
22. Sato, Y., Mori, K., Koizumi, T., Minagawa-Kawai, Y., Tanaka, A., Ozawa, E., . . . Mazuka, R. (2011). Functional lateralization of speech processing in adults and children who stutter. Frontiers in Psychology, 2, 70.
23. Foundas, A. L., Corey, D. M., Angeles, V., Bollich, A. M., Crabtree-Hartman, E., & Heilman, K. M. (2003). Atypical cerebral laterality in adults with persistent developmental stuttering. Neurology, 61(10), 1378-1385.
24. Neumann, K., Euler, H. A., von Gudenberg, A. W., Giraud, A. L., Lanfermann, H., Gall, V., & Preibisch, C. (2003). The nature and treatment of stuttering as revealed by fMRI A within- and between-group comparison. Journal of Fluency Disorders, 28(4), 381-409; quiz 409-410.
25. Neumann, K., Preibisch, C., Euler, H. A., Gudenberg, A. W. V., Lanfermann, H., Gall, V., & Giraud, A. L. (2005). Cortical plasticity associated with stuttering therapy. Journal of Fluency Disorders, 30(1), 23-39.
26. Kell, C. A., Neumann, K., von Kriegstein, K., Posenenske, C., von Gudenberg, A. W., Euler, H., & Giraud, A. L. (2009). How the brain repairs stuttering. Brain, 132 (Pt10), 2747-2760.
27. Giraud, A. L., Neumann, K., Bachoud-Levi, A. C., von Gudenberg, A. W., Euler, H. A., Lanfermann, H., & Preibisch, C. (2008). Severity of dysfluency correlates with basal ganglia activity in persistent developmental stuttering. Brain and Language, 104(2), 190-199.
28. Kang, C., & Drayna, D. (2011). Genetics of speech and language disorders. Annual Review of Genomics and Human Genetics, doi:10.1146/annurev-genom-090810-183119.
29. Newbury, D. F., & Monaco, A. P. (2010). Genetic advances in the study of speech and language disorders. Neuron, 68(2), 309-320.
30. Grigorenko, E. L. (2009). Speaking genes or genes for speaking? Deciphering the genetics of speech and language. Journal of Child Psychology and Psychiatry, 50(1-2), 116-125.
31. Maguire, G., Franklin, D., Vatakis, N. G., Morgenshtern, E., Denko, T., Yaruss, J. S., . . . Riley, G. (2010). Exploratory randomized clinical study of pagoclone in persistent developmental stuttering: the EXamining Pagoclone for peRsistent dEvelopmental Stuttering Study. Journal of Clinical Psychopharmacology, 30(1), 48-56.
32. Ingham, R. J. (2010). Comments on article by Maguire et al: pagoclone trial: questionable findings for stuttering treatment. Journal of Clinical Psychopharmacology, 30(5), 649-650; author reply 650-641.
33. Porfert, A. R., & Rosenfield, D. B. (1978). Prevalence of stuttering. Journal of Neurology, Neurosurgery and Psychiatry, 41(10), 954-956.
34. Kidd, K. K., Heimbuch, R. C., & Records, M. A. (1981). Vertical transmission of susceptibility to stuttering with sex-modified expression. Proceedings of the National Academy of Sciences, USA, 78(1), 606-610.
35. Buck, S. M., Lees, R., & Cook, F. (2002). The influence of family history of stuttering on the onset of stuttering in young children. Folia Phoniatrica et Logopaedica, 54(3), 117-124.
36. Andrews, G., Morris-Yates, A., Howie, P., & Martin, N. G. (1991). Genetic factors in stuttering confirmed. Archives of General Psychiatry, 48(11), 1034-1035.
37. Felsenfeld, S., Kirk, K. M., Zhu, G., Statham, D. J., Neale, M. C., & Martin, N. G. (2000). A study of the genetic and environmental etiology of stuttering in a selected twin sample. Behavior Genetics, 30(5), 359-366.
38. Howie, P. M. (1981). Concordance for stuttering in monozygotic and dizygotic twin pairs. Journal of Speech and Hearing Research, 24(3), 317-321.
39. Shugart, Y. Y., Mundorff, J., Kilshaw, J., Doheny, K., Doan, B., Wanyee, J., . . . Drayna, D. (2004). Results of a genome-wide linkage scan for stuttering. American Journal of Medical Genetics Part A, 124A(2), 133-135.
40. Riaz, N., Steinberg, S., Ahmad, J., Pluzhnikov, A., Riazuddin, S., Cox, N. J., & Drayna, D. (2005). Genomewide significant linkage to stuttering on chromosome 12. American Journal of Human Genetics, 76(4), 647-651.
41. Raza, M. H., Riazuddin, S., & Drayna, D. (2010). Identification of an autosomal recessive stuttering locus on chromosome 3q13.2-3q13.33. Human Genetics, 128(4), 461-463.
42. Wittke-Thompson, J. K., Ambrose, N., Yairi, E., Roe, C., Cook, E. H., Ober, C., & Cox, N. J. (2007). Genetic studies of stuttering in a founder population. Journal of Fluency Disorders, 32(1), 33-50.
43. Suresh, R., Ambrose, N., Roe, C., Pluzhnikov, A., Wittke-Thompson, J. K., Ng, M. C., . . . Cox, N. J. (2006). New complexities in the genetics of stuttering: significant sex-specific linkage signals. American Journal of Human Genetics, 78(4), 554-563.
44. Kang, C., Riazuddin, S., Mundorff, J., Krasnewich, D., Friedman, P., Mullikin, J. C., & Drayna, D. (2010). Mutations in the lysosomal enzyme-targeting pathway and persistent stuttering. New England Journal of Medicine, 362(8), 677-685.
45. Fisher, S. E. (2010). Genetic susceptibility to stuttering. New England Journal of Medicine, 362(8), 750-752.
46. Chang, S. E., Horwitz, B., Ostuni, J., Reynolds, R., & Ludlow, C. L. (2011). Evidence of left inferior frontal-premotor structural and functional connectivity deficits in adults who stutter. Cerebral Cortex, In Press.
47. Nucifora, P. G., Verma, R., Melhem, E. R., Gur, R. E., & Gur, R. C. (2005). Leftward asymmetry in relative fiber density of the arcuate fasciculus. Neuroreport, 16(8), 791-794.